Page 17 - Appetite_Stimulation_In_Dogs_Complete
P. 17
2 B. Zollers et al.
Capromorelin is an orally active small molecule that mim-
Housing
ics the action of ghrelin and is a potent and selective GHS-R
agonist, which causes appetite stimulation and GH secretion Dogs were housed individually in stainless steel cages with
just as ghrelin does. Absorption of capromorelin from the controlled temperature (18–29 °C), relative humidity (30–
gastrointestinal tract allows it to enter the circulation in the 70%), and photoperiod (12 h of light alternating with 12 h
same manner as endogenous ghrelin and naturally mimic of darkness). All dogs were under the care of a licensed
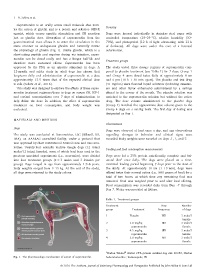
the physiology of ghrelin (Fig. 1). Unlike ghrelin, which is a veterinarian.
short-acting peptide and requires dosing via injection, capro-
morelin can be dosed orally and has a longer half-life and
Treatment groups
therefore more sustained effects. Capromorelin has been
approved by the FDA as an appetite stimulant in dogs. A The study tested three dosage regimes of capromorelin com-
12-month oral safety study in adult dogs has shown that pared to placebo treatment (see Table 1) for 7 days. Group 1
long-term daily oral administration of capromorelin at a dose and Group 4 were dosed twice daily at approximately 8 am
approximately 17.5 times that of the expected clinical dose and 6 pm (10 h 30 min apart). The placebo and test drug
is safe (Zollers et al., 2016). (31 mg/mL) were flavored liquid solutions (including sweeten-
This study was designed to explore the effects of three capro- ers and other flavor enhancers) administered by a syringe
morelin treatment regimens/doses in dogs on serum GH, IGF-1 placed in the corner of the mouth. The placebo solution was
and cortisol concentrations over 7 days of administration to matched to the capromorelin solution but without the active
help define the dose. In addition, the effect of capromorelin drug. The dose volume administered to the placebo dogs
treatment on food consumption and body weight was (Group 1) matched the approximate dose volume given to the
evaluated. Group 4 dogs on a mL/kg basis. The first day of dosing was
designated as Day 1.
MATERIALS AND METHODS
Observations
Dogs
Dogs were observed at least once a day, and any observations
The study was conducted at Xenometrics, LLC (Stilwell, KS, regarding changes in behavior and clinical signs were
USA), an AAALAC accredited facility, under a protocol that recorded. Body weights were recorded on days -1, 3, and 7.
was approved by their Institutional Animal Care and Use Com-
mittee. Twenty-four sexually mature Beagle dogs (12 intact
Feeding and food consumption measurements
males/12 intact females), some of which had been used in the
previous unrelated experiments (i.e., non-na€ ıve), were divided Dogs were fed a 25% protein nutritionally complete and bal-
into four treatment groups (n = 3 males and 3 females per anced diet a once daily. The dogs were placed on a time-
group). Dogs ranged in age from approximately 1.7–6 years. restricted feeding period beginning 7 days prior to the start of
Body weights ranged from 9–15 kg. At the end of the study, the study. At approximately 10 am ( 15 min), dogs were
all dogs were returned to the study colony. offered twice their normal ration (i.e., 800 grams of food) for a
total of two hours, at which time any remaining food was
removed. Food was weighed prior to and after food offering.
Food consumption was recorded daily from Day -7 through
Day 7. Water was provided ad libitum.
Table 1. Treatment groups
Treatment Dose Number
group Treatment frequency of dogs
1 Placebo BID 3 male/3
female
2 3.0 mg/kg SID 3 male/3
capromorelin female
3 4.5 mg/kg SID 3 male/3
capromorelin female
4 3.0 mg/kg BID 3 male/3
capromorelin female
a â
Harlan Teklad Global 25% protein certified dog diet 2025C, Harlan
Fig. 1. The pharmacologic mechanism of action of capromorelin. Laboratories Inc.; Indianapolis, IN
© 2016 The Authors. Journal of Veterinary Pharmacology and Therapeutics Published by John Wiley & Sons Ltd