Page 28 - Appetite_Stimulation_In_Dogs_Complete
P. 28
1856 Zollers et al
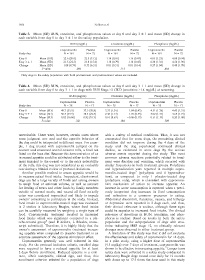
Table 3. Mean (SD) BUN, creatinine, and phosphorous values at day 0 and day 3 1 and mean (SD) change in
each variable from day 0 to day 3 1 in the safety population.
BUN (mg/dL) Creatinine (mg/dL) Phosphorus (mg/dL)
Capromorelin Placebo Capromorelin Placebo Capromorelin Placebo
Study day N = 164 N = 72 N = 164 N = 72 N = 164 N = 72
Day 0 Mean (SD) 22.6 (20.0) 22.5 (15.2) 1.18 (0.69) 1.16 (0.47) 4.03 (1.23) 4.08 (0.94)
Day 3 1 Mean (SD) 21.5 (22.3) 23.0 (15.4) 1.18 (0.79) 1.16 (0.45) 4.30 (1.33) 4.56 (1.90)
Change Mean (SD) 1.08 (8.59) 0.53 (6.21) 0.01 (0.31) 0.01 (0.14) 0.27 (1.04) 0.48 (1.78)
P-value .006 .113 .464
Only dogs in the safety population with both pretreatment and posttreatment values are included.
Table 4. Mean (SD) BUN, creatinine, and phosphorous values at day 0 and day 3 1 and mean (SD) change in
each variable from day 0 to day 3 1 in dogs with IRIS Stage ≥2 CKD (creatinine ≥1.4 mg/dL) at screening.
BUN (mg/dL) Creatinine (mg/dL) Phosphorus (mg/dL)
Capromorelin Placebo Capromorelin Placebo Capromorelin Placebo
Study day N = 28 N = 17 N = 28 N = 17 N = 28 N = 17
Day 0 Mean (SD) 49.7 (35.9) 37.5 (20.8) 2.32 (1.01) 1.84 (0.42) 4.51 (1.76) 4.45 (1.31)
Day 3 1 Mean (SD) 50.5 (39.9) 38.4 (22.4) 2.48 (1.17) 1.78 (0.45) 5.02 (1.71) 4.65 (1.75)
Change Mean (SD) 0.82 (16.86) 0.82 (9.13) 0.16 (0.61) 0.06 (0.19) 0.51 (1.19) 0.20 (1.08)
P-value .321 .184 .309
unevaluable. There were, however, certain cases where with a variety of medical conditions. Thus, it was not
some judgment was used and the appetite behavior of unexpected that for some dogs, the preexisting clinical
the dog could be interpreted in different ways. For exam- condition did not improve during the 4 days of the
ple, 1 dog treated with capromorelin jumped on the study and the dog experienced continued clinical
counter and consumed several crescent rolls, a food not decline, as evidenced in some dogs by the serious
listed on the base diet. Because this demonstration of an adverse events reported during the study. The most
increased appetite could be attributed either to the treat- common adverse reactions potentially related to treat-
ment (masked to the owner) or to the extra palatability ment were diarrhea and vomiting, which occurred with
of the rolls, this dog was not considered as an evaluable similar frequencies in both treatment groups. Polydipsia
case during a blinded review of protocol deviations. and hypersalivation were reported in multiple capro-
Another possible method to assess appetite would be morelin-treated dogs 7 (polydipsia and 4 hypersaliva-
to measure actual food intake. Inappetent, client-owned tion), as compared to only 1 placebo-treated dog
dogs are fed a large variety of foods and asking owners (polydipsia only), which is consistent with capromore-
to weigh food or estimate volume, as well as account lin’s mechanism of action to increase appetite: An
for feeding of treats and other foods by family members increase in water consumption is expected when a previ-
outside of normal meals, and record all food actually ously inappetent dog increases its food consumption
eaten was considered impractical and likely to impede and salivation is a common precursor to eating.
owners’ consenting to participate in the study. Instead, Abdominal discomfort, flatulence, nausea, and lethargy
food intake in capromorelin-treated dogs was evaluated or depression were reported in 2 dogs each, and thus,
in 2 studies in laboratory Beagles, where food intake their clinical relevance is unclear, although these signs
can be carefully measured by weighing a single type of could be sequelae to increased food intake. The clinical
food. Capromorelin treatment resulted in increased relevance of elevations in BUN, creatinine, and phos-
food intake which was significant compared to a pla- phorus is unclear. Based on results in both the safety
6
cebo-treated group (and B Zollers, L Rhodes, manu- population and the subpopulation of dogs with crea-
script submitted). Therefore, it is highly likely that the tinine indicating IRIS stage ≥2, no clinically relevant
owner’s assessment of capromorelin treatment increas- changes were observed in either treatment group.
ing appetite when compared to placebo treatment is an A limitation of this study was that it did not evaluate
accurate assessment of the appetite stimulation effect. the effectiveness and safety of capromorelin for >4 days.
Capromorelin generally was well tolerated in this In a pilot study, using a higher dose of capromorelin
study. Per the protocol inclusion criteria, enrolled dogs (4.5 mg/kg) over 7 days of treatment, capromorelin was
7
were required to have a reduced appetite for at least shown to be effective in increasing appetite. Longer term
2 days before enrollment. Reduced appetite is a com- safety data were collected in a safety study conducted in
mon, albeit nonspecific, clinical sign, and thus, this laboratory Beagle dogs treated once daily for 12 months
enrollment criterion selected for a population of dogs with doses up to 17.5 times the dose used in this study